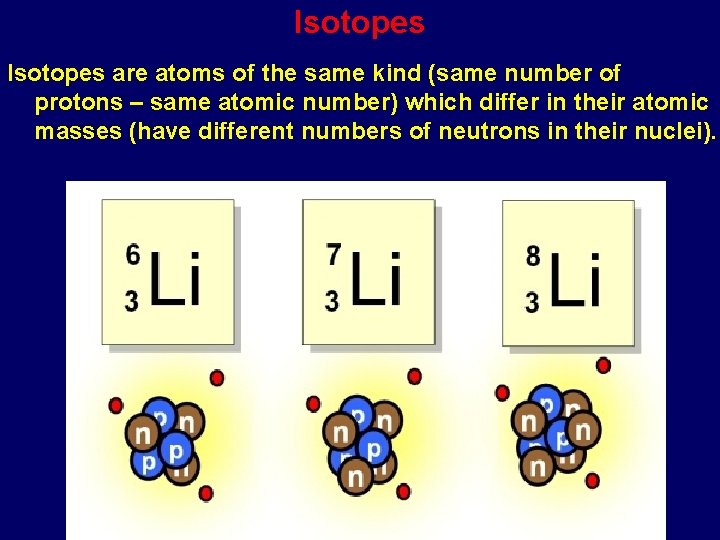
Isotopes are variants of a particular chemical element which differ in neutron number, and consequently in nucleon number. Shazam online music recognition. Adrienne miller in the land of men &. All isotopes of a given element have the same number of protons but different numbers of neutrons in each atom. Isotope any of two or more versions of a chemical element, having the same number of protons in the nucleus, or the same atomic number, but having different numbers of neutrons in the nucleus, or different atomic masses.
 Also found in: Thesaurus, Medical, Encyclopedia, Wikipedia.
Also found in: Thesaurus, Medical, Encyclopedia, Wikipedia.Isotopes Are Formed When
Related to isotopes: Radioactive isotopes
isotopes
1. Atoms of the same element (all chemically identical) having the same atomic number but containing different numbers of neutrons, giving a different mass number.
2. Atoms of an element with an identical number of protons but differing numbers of neutrons.
Dictionary of Unfamiliar Words by Diagram Group Copyright © 2008 by Diagram Visual Information Limited

Want to thank TFD for its existence? Tell a friend about us, add a link to this page, or visit the webmaster's page for free fun content.
Isotopes Are Dangerous
What Is True About Isotopes
Link to this page: